
It makes sense to complete the review in a timely manner for budget input. The output of the review is to set goals and objectives for the forthcoming period, so it is good practice to align the review period with the organization’s financial year, parent company strategy sessions, and the laboratory’s accreditation assessments. To meet the intent of the review, typically, at least one review of the entire management system should be performed each year. Responsibility is usually assigned to the top laboratory manager, who will conduct a final review with the gathered information and contributions. The management review can be conducted over a number of meetings with previously written agendas, where partial inputs may even be evaluated via email or other collaborative platforms at different levels within the laboratory. To clarify a misconception about management reviews, note that the review doesn’t have to be a singular annual meeting covering all requirements. How often, how, and when should management review be conducted? Evaluate the best use of resources – the relationship between the results achieved and the resources used, i.e., doing things right. Evaluate the extent to which the management system is capable of accomplishing the purpose of producing the intended or expected result, i.e., doing the right things.
#Iso 17025 2017 management review template iso#
Evaluate if all the mandatory ISO 17025 contractual, organizational, and regulatory requirements are met. Are the correct processes and operations included to enable/support the activities of the laboratory? There are, therefore, four key parameters that are considered during management review in ISO/IEC 17025: When shortcomings are identified through management review, provisions can be made for necessary changes and required resources.Įfficiency should also be evaluated to ensure best use of allocated resources. Organizations and stakeholders (including the accreditation body) need to be assured of the continuing suitability, adequacy, and effectiveness of the laboratory’s management system, along with its stated policies and objectives. Why is it important to perform management reviews? To learn more about the maintenance of the ISO 17025 management system, read this: Maintaining and improving quality management in laboratories according to ISO 17025:2017. The purpose of a management review is for top management and personnel involved in the decision-making processes to systematically evaluate the overall performance of the laboratory and its Quality Management System. Management review is a strategic planning opportunity. It is not about reporting measurements, but evaluating to what extent the management system fulfills its functions and goals.

Management review is neither an audit nor a report-back discussion.

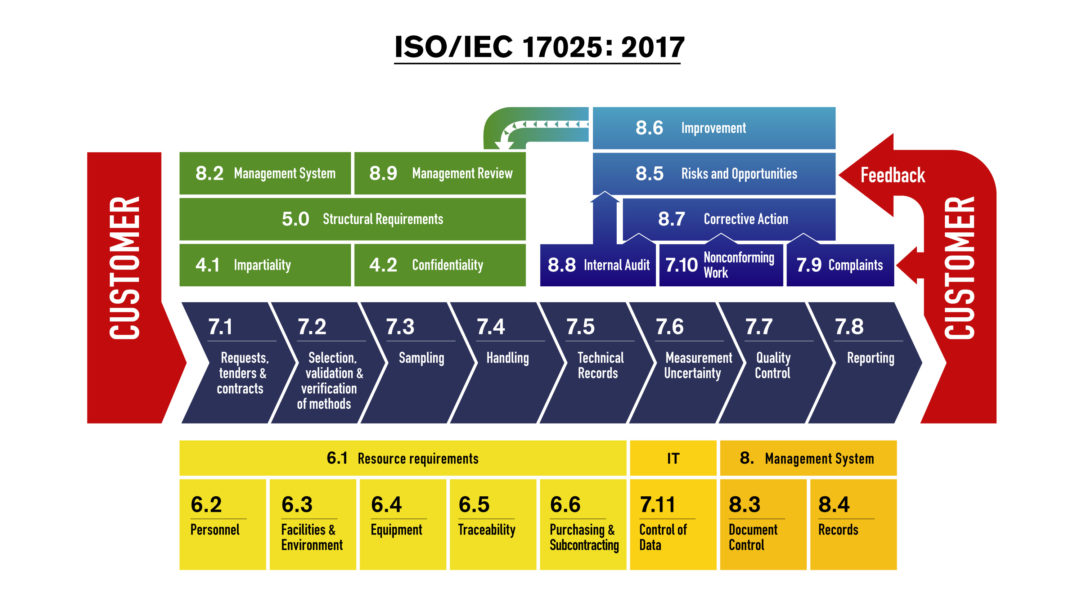
#Iso 17025 2017 management review template how to#
It will provide some practical guidance on how to go about the management review and how to structure your documents to meet ISO 17025:2017 clause 8.9 requirements. This article will help you gain a better understanding of what management review (MR) means, as well as its importance and value.


 0 kommentar(er)
0 kommentar(er)
